
Table of
Contents
 Table of Contents |
a. This chapter will cover basic biophysical and biological effects of ionizing radiation in order to form a foundation for understanding the clinical aspects of radiation injury discussed in Section IV of Chapter 6. This extended discussion of radiation does not imply that nuclear radiation will be the most important cause of casualties after a nuclear explosion. Blast and thermal injuries in many cases will far outnumber radiation injuries. However, radiation effects are considerably more complex and varied than are blast or thermal effects and are subject to considerable misunderstanding. As a result, a more detailed discussion is warranted. Since data from human experience are limited, much of the information in this chapter is based upon experimental information from animal studies.
b. A wide range of biological changes may follow the irradiation of an animal, ranging from rapid death following high doses of penetrating whole-body radiation to an essentially normal life for a variable period of time until the development of delayed radiation effects, in a portion of the exposed population, following low dose exposures. The nature and severity of these changes will depend upon a great variety of biological and physical factors. There are significant variations in response to irradiation associated with differences in species, age, and other biological factors, as well as the physical factors of dose, dose rate, or nature of the radiation. However, the biological responses to radiation are not unique. They fall within the range of standard tissue responses seen following other types of injury and occur as a result of similar biochemical and/or cell kinetic disturbances. As a result, the wide range of effects which is possible can be organized into a predictable scheme, the details of which form the basic material of this chapter.
A wide variety of ionizing radiation can interact with biological systems, but there are only four types of radiation associated with atmospheric and underground nuclear detonations of biological significance. In order of importance, they are gamma, neutron, beta, and alpha. The physical natures of these are discussed at length in Chapter 2. However, certain aspects of their mechanisms of interaction with living tissue are summarized here.
a. Gamma radiation, emitted during the nuclear detonation or later in fallout, is highly energetic and is so penetrating that a significant part will pass through the human body without interaction. About 75% of the photons will interact with and lose energy to the atoms of the target tissue. This energy deposition may occur anywhere along a given photon's path, and therefore, anywhere in the body. If the gamma photon flux is high and the whole body is exposed, a fairly homogeneous deposition of energy will occur. This is in marked contrast to the highly localized energy deposition patterns of alpha and beta radiations.
b. Because of its penetrating ability, the effects of gamma irradiation can be independent of the location of the source, (i.e., internal or external to the body). High-energy gamma emitters deposited within the body can result in total body irradiation just as effectively as external sources, if the quantities deposited are large enough and despite the fact that the emitters may not be distributed uniformly throughout the body.
a. Neutron Interaction.
(1) Since neutrons are uncharged particles and can react only with the nuclei of target atoms, the probability of interaction of neutrons in the energy range characteristic of the fission spectrum detonation during their path through the human body is roughly comparable to that of low-energy gamma photons. Therefore, neutron radiation can result in whole-body irradiation. The energy deposition will not be uniform, and the side of the body which faces the detonation will absorb more energy than the opposite side. However, this difference, although of great theoretical interest, is not of operational importance. The major effect of this nonuniform deposition of energy will be to cause a wide variation in the typical radiation doses causing radiation sickness rather than significant variation in the overall clinical effects.
(2) As noted above, neutrons, since they are uncharged neutral particles, do not interact with the orbital electrons of atoms as do other forms of radiation. Instead, they interact with atomic nuclei directly. Because of their mass and energy, neutrons can cause severe disruptions in atomic structure, typically causing a recoil "escape" of a target nucleus from its orbital electrons. This is much more common with the very light atoms, particularly hydrogen, since the mass of the photon making up the nucleus of common hydrogen is the major target atom in living tissue. When the nuclei of these latter are accelerated they are capable of causing dense ionization along their paths.
(3) In biological material, elastic collisions of this type between neutrons and the nuclei of light-weight atoms predominate. Due to their short range, the accelerated nuclei produced by these collisions will expend their energy along short tracks of high excitation and ionization density. In tissue, about 70% to 85% of the entire fast neutron energy is transferred to recoil hydrogen nuclei. The remainder of the neutron energy is dissipated in recoil nuclei of the other atoms noted above.
(4) After the neutrons have lost most of their energy through these collisions, they will reach an equilibrium energy state in which they are referred to as thermal neutrons. Such relatively slow moving neutrons have a high probability of being captured by the nuclei of a wide variety of elements such as sodium. The resulting materials are radioactive and generally decay rapidly. The resulting tissue irradiation is not a significant factor in radiation injury since the total energy released by the decay of these radioactive materials is extremely small compared to the total energy absorbed from the neutrons by elastic collisions. However, the quantities can be measured and can be used to estimate neutron doses in limited numbers of casualties.
b. Neutron Relative Biological Effectiveness.
(1) Relative biological effectiveness represents the effectiveness of a given radiation, compared to a reference radiation, (250 kilovolts (Kvp) x-rays), in producing the same level of response. Relative Biological Effectiveness (RBE) is defined as the ratio of the absorbed dose of a reference radiation to the absorbed dose of a test radiation to produce the same level of biological effect, other conditions being equal. (See Table 5-1.) When two radiations produce a biological effect that is not of the same extent and/or nature, the RBE cannot be specified.
(2) Marked changes in behavior, vomiting, cardiovascular disorders, neurological symptoms, and other symptoms have been observed in monkeys irradiated at doses between 0.5 and 6.5 gray (Gy) by a fission neutron flux with neutron dose/gamma dose ratios varying from 1 to 12 and a dose rate close to those delivered by "conventional" nuclear weapons.
(3) It was found that the neutron RBE (fission spectrum neutrons) for these disturbances was approximately between 0.5 and 1.2 in the range from 0.5 to 6.5 Gy. These RBE values must be confirmed by using a gamma radiation source with a dose rate comparable to that delivered by the reactors used and compared with those which would be obtained with neutrons from a fusion weapon. The above results lay particular stress upon the importance of intermediate dose and the biological effects of these as causes of incapacity can no longer be regarded as insignificant. In operational terms, neutron RBE varies with neutron energy, with neutron dose (the size of the neutron dose/gamma dose ratio), the dose rate and above all the dose gradient, particularly for determination of hematological LD50, but doubtless also for vomiting and early transient incapacitation (ETI). The RBE for ETI has been established as being equal to 1, because insufficient evidence has been collected to indicate otherwise. Relating dose to radiation effects in humans and other large mammals is further complicated by the fact that mixed-spectrum radiations change as they interact with body tissue. This change in quality of a mixed-spectrum field is significant since the biological damages produced by high-LET and low-LET radiations are not equivalent. High-LET radiations such as alpha particles or fast neutrons are generally regarded to have a greater relative biological effectiveness than low-LET radiations such as x-rays and gamma photons. The one exception to this generalization that seems to be significant in predicting the effects of ionizing radiation on combat personnel is that gamma photons have been found to be more effective in producing early transient incapacitation than either high-energy neutrons or fission spectrum neutrons.
a. High speed electrons in the form of beta radiation lose most of their energy after penetrating only a few millimeters of tissue. If the beta emitting material is on the surface of the skin, the resulting beta irradiation causes damage to the basal stratum of the skin. The lesion is similar to a superficial thermal burn. However, if the beta material is incorporated internally, the beta radiation can cause much more significant damage. The damage will be in spheres of tissue around each fragment or source of radioactive material. The total damage is a function of the number of sources and their distribution in the body. The distribution is determined by the chemical nature of the material.
b. Table 5-II lists the critical ranges of radiation exposure in tissue for beta emitters of various energies. These ranges are considerably greater than those for alpha particles (Table 5-III). In addition to a difference in range when compared with alpha radiation, there is also a significant difference in the pattern of energy deposition. The density of energy deposited is much less for beta irradiation than for alpha, and as a result, the target cells may be damaged rather than killed outright. Damaged cells may be of greater significance to the total organism than killed cells, particularly if they go on to become malignant or otherwise malfunction. Killed cells are replaced quickly in most tissues with any degree of reserve capacity and do not cause significant overall clinical effects unless the cells involved are highly critical or the fraction of cells killed in a given organ is large.
a. The energy of these relatively heavy, positively charged particles is fully absorbed within the first 20 micrometers of an exposed tissue mass. If the source of the radiation is external, all of the alpha radiation is absorbed in the superficial layers of dead cells within the stratum corneum. If anything, even tissue paper, is interposed, the alpha particles will be absorbed, and not reach the skin. Because of this, alpha radiation is not an external hazard. If alpha emitting material is internally deposited, all the radiation energy will be absorbed in a very small volume of tissue immediately surrounding each particle. Alpha radiation has such limited penetrating ability that the maximum range for the highest energy alpha particle in tissue is less than 100 micrometers. Thus, while extremely high radiation doses may be deposited in the few cells immediately surrounding a source of alpha radiation, regions outside this small irradiated spherical volume are not affected. Table 5-III illustrates this for a 37 KBq (1.0 �Ci) source of an alpha emitter of moderate energy.
b. Beyond a radius of about 20 micrometers, the deposition of energy is very small. Due to the high radiation doses within this critical radius, the cells immediately adjacent to the source are killed. They would then be removed by phagocytosis or replaced by fibrosis. Relatively little damage to the intact organism results, unless these cells are themselves highly critical. Most tissues with a reasonable reserve can tolerate the loss of a few cells quite readily, particularly if the tissues have a normally high turnover rate. Therefore, although internal alpha radiation can be lethal to individual cells, the overall acute hazard is small. Internal deposition of alpha particles are of importance on a long term basis in terms of causing radiation injury which is of greater significance than from beta particles. However, injury from internal deposition of alpha particles is not of military importance.
c. However, many alpha emitting materials also emit gamma radiation, and this gamma radiation may cause significant tissue injury, even though the total alpha energy exceeds the total gamma energy and the ratio of gamma emissions per alpha is very small. This follows from the fact that the penetrating power of gamma radiation is many times greater than that for alpha radiation so that the total volume of tissue exposed to damaging radiation is many times greater.
a. When radiation interacts with target atoms, energy is deposited, resulting in ionization or electron excitation as described in Chapter 2. This ionization or excitation must involve certain critical molecules or structures in a cell in order that the damage caused by radiation may follow the consistent patterns it does. It has been theorized that this localization of absorbed energy in critical molecules could be either a direct or an indirect action, i.e., the energy deposited by the radiation may involve particular sensitive chemical bonds directly, or it may be deposited elsewhere first and transferred to the sensitive bonds by means of an appropriate energy transfer system. The former mechanism implies that the radiation quite precisely hits particular target atoms, whereas the latter implies that there is a method for preferentially directing randomly deposited energy to sensitive sites.
b. The exact radiochemical mechanism involved in mammalian systems subjected to whole-body doses of penetrating radiation is not fully understood. However, the most reasonable hypothesis at the present time is that water, both intracellular and extracellular, is the primary site of radiation energy deposition and that the energy deposited in the water would be transferred to and affect sensitive molecules indirectly.
Observed cellular effects of radiation, whether due to direct or indirect damage, are basically similar for different kinds and doses of ionizing radiation.
a. Cell Death. One of the simplest effects to observe is cell death, the course of which can be described by various terms.
(1) Pyknosis. The nucleus becomes contracted, spheroidal, and filled with condensed chromatin.
(2) Karyolysis. The nucleus swells and loses its chromatin.
(3) Protoplasmic Coagulation. Irreversible gelatin formation occurs in both the cytoplasm and nucleus.
(4) Karyorrhexis. The nucleus becomes fragmented and scattered throughout the cell.
(5) Cytolysis. Cells swell until they burst and then slowly disappear.
b. Changes in Cell Function. Nonlethal changes in cellular function can occur as a result of lower radiation doses. These include delays in certain phases of the mitotic cycle, disrupted cell growth, permeability changes, and changes in motility.
(1) Mitotic Cycle. Mitosis may be delayed or inhibited following radiation exposure. Dose dependent inhibition of mitosis is particularly common in actively proliferating cell systems. This inhibition occurs approximately 40 minutes before prophase in the mitotic cycle, at a time when the chromosomes are discrete, but prior to the breakdown of the nuclear membrane. Subsequent irradiation after this radiation transition point does not delay mitosis. Delays in mitosis can cause profound alterations in cell kinetic patterns resulting in depletions of all populations. This is the basic kinetic patterns resulting in depletions of all populations. This is the basic mechanism underlying the later clinical changes seen in the hematopoietic and gastrointestinal syndromes of whole-body irradiation.
(2) Disruptions in Cell Growth. Cell growth may also be retarded, usually after a latent period. This may be due to progressive formation of inhibitory metabolic products and/or alterations in the cell microenvironment.
(3) Permeability Changes. Irradiated cells may show both increased and decreased permeability. Radiation changes within the lipid bilayers of the membrane may alter ionic pumps. This may be due to changes in the viscosity of intracellular fluids associated with disruptions in the ratio of bound to unbound water. Such changes would result in an impairment of the ability of the cell to maintain metabolic equilibrium and could be very damaging even if the shift in equilibrium were quite small.
(4) Changes in Cell Motility. The motility of a cell may be decreased following irradiation. However, the presence of normal motility does not imply the absence of radiation injury. Irradiated spermatozoa, for example, may retain their motility and be capable of fertilization while carrying radiation-induced genetic changes which may alter subsequent embryogenesis.
In general, actively proliferating cells are most sensitive to radiation. On the other hand, the mitotic activity of all cells decreases with maturation. Thus, cellular radiosensitivity tends to vary inversely with the degree of differentiation. Cells may be classified functionally and in decreasing order of sensitivity into four categories: vegetative cells, differentiating cells, totally differentiated cells, and fixed non-replicating cells.
a. Vegetative Cells. These cells, comprising differentiated functional cells of a large variety of tissues, are generally the most radiosensitive. Examples include:
(1) Free stem cells of hematopoietic tissue (hemocytoblasts, primitive lymphoblasts, primitive erythroblasts, and primitive myeloblasts).
(2) Dividing cells deep in the intestinal crypts.
(3) Primitive spermatogonia in the epitheliums of the seminiferous tubules.
(4) Granulosa cells of developing and mature ovarian follicles.
(5) Basal germinal cells of the epidermis.
(6) Germinal cells of the gastric glands.
(7) Large and medium sized lymphocytes.
(8) Small lymphocytes, which are not included normally in this class of cells, but which are also highly radiosensitive.
(9) Mesenchymal cells.
b. Differentiating Cells. These cells are somewhat less sensitive to radiation. They are relatively short-lived and include the first generation produced by division of the vegetative mitotic cells. They usually continue to divide a limited number of times and differentiate to some degree between divisions. As differentiation occurs, radiosensitivity decreases. The best examples of this type of cell are the dividing and differentiating cells of the granulocytic and erythrocytic series in the bone marrow. This type also includes the more differentiated spermatogonia and spermatocytes in the seminiferous tubules and the ovocytes.
c. Totally Differentiated Cells. These cells are relatively radioresistant. They normally have relatively long lifespans and do not undergo regular or periodic division in the adult stage, except under abnormal conditions such as following damage to or destruction of a large number of their own kind. This class includes hepatocytes, cells of interstitial gland tissue of the gonads, smooth muscle cells, and vascular endothelial cells.
d. Fixed Nonreplicating Cells. These cells are most radioresistant. They do not normally divide, and some types, such as neurons, do not divide under any circumstances. They are highly differentiated morphologically and highly specialized in function. Cells in this group have widely varied life-spans and show progressive aging. This group includes the long-lived neurons, striated muscle cells, short-lived polymorphonuclear granulocytes and erythrocytes, spermatids and spermatozoa, and the supeficial epithelial cells of the alimentary tract.
The relative sensitivity of an organ to direct radiation injury depends upon its component tissue sensitivities. Table 5-IV lists various organs in decreasing order of radiosensitivity on the basis of a relatively direct radiation effect, parenchymal hypoplasia.
a. Cell nuclei contain chromosomes which in turn contain the genes controlling cellular somatic and reproductive activity. These chromosomes are composed of deoxyribonucleic acid (DNA), the macromolecule containing the genetic information. This is a large, tightly coiled, double-stranded molecule and is sensitive to radiation damage. Radiation effects range from complete breaks of the nucleotide chains of DNA, to point mutations which are essentially radiationinduced chemical changes in the nucleotides which may not affect the integrity of the basic structure. Intermediate effects, such as abnormal bonding between adjacent molecules and alterations in viscosity, have also been observed.
b. After irradiation, chromosomes may appear to be "sticky" with formation of temporary or permanent interchromosomal bridges preventing normal chromosome separation during mitosis and transcription of genetic information. Unequal division of nuclear chromosome material between daughter cells with production of nonviable abnormal nuclei may result.
Laboratory studies in animals indicate increased mutation rates with small doses of radiation. As radiation dose increases, mutation induction also increases. Mutations per unit dose decrease at low dose rates. However, viable mutations are still extremely rare. Most of the mutations are lethal and thus self-limiting. It must be kept in mind that radiation doses increase natural mutation rates and that the mutations produced, and not visibly detected, are permanent in regard to future generations.
a. Each of the numerous cell renewal systems making up an animal's total cellular mass is normally in an equilibrium state between cell formation, proliferation, maturation, and death. Some systems, such as the adult central nervous system in higher animals, are stabilized at the end point of maturation, and the functional cells of such a system are not replaced if lost or destroyed. Other organ systems, such as the liver, which do not normally replace cells at a rapid rate, have the potential to regenerate large numbers of cells if needed. Other organ systems, such as the skin, the reproductive system, the gastrointestinal tract, and the hematopoietic system in the bone marrow, maintain a continuous high cell turnover rate. Bone marrow also has a large reserve capacity in the adult. A large fraction of it is normally nonfunctioning but has the potential to be functional if required. Failure of a particular organ system may or may not lead to death of the animal, depending on the importance of that system's functions, i.e., failure of gonadal function would not be lethal, whereas failure of bone-marrow function would be.
b. Regardless of the biophysical processes involved, one of the major biological effects of whole-body radiation, in the dose ranges causing the syndromes of bone-marrow depression and gastrointestinal damage, is a profound disturbance in the cell kinetics of these systems. Both the hematopoietic and the gastrointestinal system have fairly rapid cellular replacement rates and normally contain cell populations in all stages of maturation and differentiation from primitive stem cells to mature functional cells.
c. The stem cells of the various cell lines of these systems are almost all relatively sensitive to radiation whereas the mature functional cells are relatively resistant. As a result, following radiation, injured stem cells are not likely to mature. When the mature cells die or are otherwise lost they will not be replaced and the overall population of cells in the system will be decreased. If the radiation injury is repairable, recovery of the ability of a stem cell population to mature will result in a gradual return of a mature, functional population. If the damage is irreversibly severe, there will be no recovery.
The bone marrow contains three cell renewal systems: the erythropoietic (red cell), the myelopoietic (white cell), and the thrombopoietic (platelet). The time cycles and cellular distribution patterns and postirradiation responses of these three systems are quite different.
a. Studies suggest that a phuripotential stem cell gives rise to these three main cell lines in the bone marrow. Beyond this stem cell, each cell renewal system consists of a stem cell compartment for the production of erythrocytes, leukocytes (lymphocytes, granulocytes, monocytes, etc.), or platelets, a dividing and differentiating compartment, a maturing (nondividing) compartment, and a compartment containing mature functional cells.
b. Research studies suggest that each of these cell renewal systems operates under the influence of regulating factors, primarily at the stem cell level, through a negative feedback system initiated in large measure by the level of mature circulating cells in the peripheral blood. Normally, a steady-state condition exists between new cell production by the bone marrow and the numbers of functional cells. Morphological and functional studies have shown that each cell line, i. e., erythrocyte, leukocyte, and platelet, has its own unique renewal kinetics. The time-related responses evident in each of these cell renewal systems after irradiation are integrally related to the normal cytokinetics of each cell system.
a. The function of this cell renewal system is to produce mature erythrocytes for the circulation. The transit time from the stem cell stage in the bone marrow to the mature red cell ranges from 4 to 7 days, after which the life-span of the red cell is approximately 120 days. The immature forms, i.e., erythroblast and proerythroblast, undergo mitosis as they progress through the dividing and differentiating compartment. Because of their rapid proliferating characteristics they are markedly sensitive to cell killing by ionizing radiation. Cell stages within the maturing (nondividing) and functional compartments, i.e., normoblast, reticulocyte, and red cell, are not significantly affected by midlethal to lethal range doses. The death of stem cells and of those within the next compartment is responsible for the depression of erythropoietic marrow and, if sufficiently severe, is responsible together with hemorrhage for subsequent radiation-induced anemia. Because of the relatively slow turnover rate, e.g., approximately 1 percent loss of red cell mass per day, in comparison with leukocytes and platelets, evidence of anemia is manifested subsequent to the depression of the other cell lines, provided that significant hemorrhage has not occurred.
b. The erythropoietic system has a marked propensity for regeneration following irradiation from which survival is possible. After sublethal exposures, marrow erythropoiesis normally recovers slightly earlier than granulopoiesis and thrombopoiesis and occasionally overshoots the base-line level before levels at or near normal are reached. Reticulocytosis, occasionally evident in peripheral blood smears during the early intense regenerative phase occurring after maximum depression, often closely follows the temporal pattern of marrow erythropoietic recovery. Although anemia may be evident in the later stages of the bone-marrow syndrome, it should not be considered a survival-limiting sequela.
a. The function of the myelopoietic marrow cell renewal system is mainly to produce mature granulocytes, i.e., neutrophils, eosinophils, and basophils, for the circulating blood. Of these, the neutrophils, because of their role in combatting infection, are the most important cell type in this cell line. The stem cells and those developing stages within the dividing and differentiating compartment are the most radiosensitive. These include the myeloblast, progranulocyte and myelocyte stages. As with the erythropoietic system, cell stages within the maturing (nondividing) compartment and the mature functional compartment, i.e., granulocytes, are not significantly affected by radiation doses within the midlethal range. Three to seven days are normally required for the mature circulating neutrophil granulocyte to form from its stem cell precursor stage in the bone marrow.
b. Mature functional granulocytes are available upon demand from venous, splenic and bone-marrow pools. Following an initial increase in circulating granulocytes (of unknown etiology), these pools are normally depleted before granulocytopenia is evident soon after radiation-induced bone-marrow injury. Because of the rapid turnover of the granulocyte cell renewal system due to the short life-span of its cells (approximately 8 days), evidence of radiation damage to marrow myelopoiesis occurs in the peripheral blood within 2 to 4 days after whole-body irradiation. The brief latent period between the time of irradiation and the beginning depletion of circulating granulocytes is related to the transit time of the nonradiosensitive cells within the nondividing, maturing marrow compartment, i.e., metamyelocyte and band forms, during their development into mature circulating granulocytes. Maturation depletion of these stages in the absence of feed-in of the earlier radiosensitive stages damaged by radiation accounts for the granulocytopenia.
c. Recovery of myelopoiesis lags slightly behind erythropoiesis and is accompanied by rapid increases in numbers of differentiating and dividing forms in the marrow. Prompt recovery is occasionally manifested and is indicated by increased numbers of band cells in the peripheral blood.
a. The thrombopoietic cell renewal system is responsible for the production of platelets (thrombocytes) for the peripheral circulating blood. Platelets along with granulocytes constitute two of the most important cell types in the circulation, the levels of which during the critical phase after midlethal doses will markedly influence the survival or nonsurvival of irradiated personnel. Platelets are produced by megakaryocytes in the bone marrow. Both platelets and mature megakaryocytes are relatively radioresistant; however, the stem cells and immature stages are very radiosensitive. During their developmental progression through the bone marrow, megakaryocytic precursor cells undergo nuclear division without cell division. The transit time through the megakaryocyte proliferating compartment in humans ranges from 4 to 10 days. Platelets have a life-span of 8 to 9 days.
b. Although platelet production by megakaryocytes may be reduced by a high dose of ionizing radiation, the primary effect is on stem cells and immature megakaryocyte stages in the bone marrow. As with the erythropoietic and myelopoietic systems, the time of beginning depression of circulating platelets is influenced by the normal turnover kinetics of cells within the maturing and functional compartments. Early platelet depression, reaching thrombocytopenic levels by 3 to 4 weeks after midlethal range doses, occurs from killing of stem cells and immature megakaryocyte stages and from maturation depletion of maturing and functional megakaryocytes.
c. Regeneration of thrombocytopoiesis after sublethal irradiation normally lags behind both erythropoiesis and myelopoiesis. Supranormal platelet numbers which overshoot the preirradiation level have occurred during the intense regenerative phase in human nuclear accident victims. The mechanism of the prompt rapid recovery of platelet numbers after acute sublethal irradiation may be explained by the response of the surviving and regenerating stem cell pool to a human feedback stimulus from the acute thrombocytopenic condition. Accelerated differentiation and maturation of immature megakaryocytes as well as marked increases in size of megakaryocytes contribute to the intense platelet production and eventual restoration of steady-state levels. Blood coagulation defects with concomitant hemorrhage constitute important clinical sequelae during the thrombocytopenic phase of bone-marrow and gastrointestinal syndromes.
In view of the vulnerability of the small intestine to radiation damage and the important role it plays in the gastrointestinal syndrome, the cell renewal kinetics of the villi of this segment are important.
a. The renewal system is in the crypt and villus where epithelial cell formation, migration and loss occur. The four cell renewal compartments are: stem cell and proliferating cell compartment, maturation compartment, functional compartment, and the extrusion zone. Stem cells and proliferating cells move from crypts into a maturing only compartment at the neck of the crypts and base of the villi. Functionally mature epithelial cells than migrate up the villus wall and are extruded at the villus tip. The overall transit time from stem cell to extrusion on the villus for humans is estimated as being 7 to 8 days. Shorter times for epithelial cell renewal systems have been reported in experimental animals.
b. Because of the high turnover rate occurring within the stem cell and proliferating cell compartment of the crypt, marked damage occurs in this region by whole-body radiation doses above the midlethal range. Destruction as well as mitotic inhibition occurs within the highly radiosensitive crypt and proliferating cell compartments within hours after high doses. Maturing and functional epithelial cells continue to migrate up the villus wall and are extruded albeit the process is slowed. Shrinkage of villi and morphological changes in mucosal cells, i.e., columnar to cuboidal to squamoid, occur as new cell production is diminished within the crypts. Continued extrusion of epithelial cells in the absence of cell production can result in denudation of the intestinal mucosa. Concomitant injury to the microvasculature of the mucosa and submucosa in combination with epithelial cell denudation results in hemorrhage and marked fluid and electrolyte loss contributing to shock. These events normally occur within 1 to 2 weeks after irradiation. A second mechanism of injury has recently been detected at the lower range of the gastrointestinal syndrome, or before major denudation occurs at higher doses of radiation. This response is a functional increase in fluid and electrolyte secretion on the epithelial cells without visible cell damage. This second mechanism may have important implications for fluid replacement therapy. Other secondary complications which contribute significantly to the gastrointestinal syndrome will be described elsewhere.
Whole-body irradiation is the most important type of radiation exposure since it is the most damaging and is discussed in the greatest detail in this section. However, partial body and specific organ irradiation can occur, particularly from internal deposition and retention of radioactive fission products found in fallout. Basic biophysical principles of internal irradiation are also discussed in a later section of this chapter. Severe radiation sickness is seen following large dose of external whole-body irradiation. Variable lesser degrees of radiation sickness may occur following partial body irradiation. The mechanisms underlying the various syndromes of severe radiation sickness are emphasized in this section.
a. Lethality.
(1) When comparing the effects of various types or circumstances, that dose which is lethal to 50% of a given population is a very useful parameter. The term is usually defined for a specific time, being limited, generally, to studies of acute lethality. The common time periods used are 30 days or less for most small laboratory animals and to 60 days for large animals and humans. On occasion, when a specific type of death is being studied, the time period used will be shorter. The specified period of time is indicated by a second number in the subscript; LD50/30 and LD50/5 indicate 50% mortality within 30 days and 5 days, respectively. The LD50 is a median; the easiest method of approximating it is by plotting experimental data on an appropriate graph and then estimating it by inspection. It should be understood that the LD50/60 assumes that the individuals did not receive other injuries or medical treatment.
(2) Figure 5-I is a graphic representation of a typical mortality response to radiation. The curve drawn through the data points is sigmoid, indicating that the mortality response to increasing dose approximates a normal distribution. A sigmoid curve is difficult to plot, particularly when the number of data points is limited, and a preferred method which allows the plotting of experimental data along a straight line is used in most mortality studies. Figure 5-II shows the same experimental data plotted on a specially constructed graph, termed a probit graph. This distortion is deliberate and is based upon the function of a normal distribution, so that data from a normal distribution can be plotted on such a graph, which seems to fit the data. The LD50 can then be estimated by inspection. This method is simple and used extensively. However, it should only be used when it has been demonstrated that the dose response phenomenon being studied does indeed follow or at least reasonably approximates a normal distribution. There is controversy about what the LD50/60 for humans is. A full discussion of this issue is beyond the scope of this overview.
(3) Medically, other figures of interest are the dose that will kill virtually no one, (LD5), and the dose that will kill virtually every one (LD95). Approximations of those doses are within the ranges 200-300 cGy (free in air) and 600-700 cGy (free in air), respectively.
b. Radiation-Induced Early Incapacitation.
(1) The focus of animal studies has been the incapacitation of the subhuman primate, since the incapacitation response has military relevance and the response of primates seems most like a human's response after acute wholebody irradiation. For high radiation doses (in excess of 1000 cGy), early transient incapacitation (ETI) occurs on average within 5 to 10 minutes after acute whole-body irradiation. With lowering the dose the median time of ETI occurrence increases up to 12 to 15 minutes. Typical duration of ETI is of the order of 15 minutes. Performance decrement in the monkey has been evaluated for numerous behavioral takes after whole-body and partial-body irradiation for various radiation qualities and dose rates. Several generalizations have emerged from these studies.
(a) Early transient incapacitation is qualitatively very similar for many behavioral tasks.
(b) The frequency function of radiation of incapacitation within a population increases as a dose.
(c) Incapacitation can be elicited by either trunk-only or head-only irradiation.
(d) Neutrons are less effective in producing early transient incapacitation than are gamma photons. The relative biological effectiveness for incapacitation of neutrons to gammas has been estimated between 0.23 and 0.62.
(e) The frequency of incapacitation produced by a given radiation dose is proportional to the demands or stress of the task being performed. These findings and the data they represent are the basis for the current combat casualty criteria. The present criteria are based on the incapacitating dose levels for both physically demanding tasks and undemanding tasks. They do not include combat ineffectiveness due to partially degraded performance that may result from slower reaction to the task, task stress, or prodromal effects of the acute radiation sickness.
(2) For yields of 5-10 Kt (or less), initial nuclear radiation is the dominant casualty producer on the battlefield. Military personnel receiving an acute incapacitation dose (30 Gy) will become performance degraded almost immediately and combat ineffective within several hours. However, they will not die until 5-6 days after exposure if they do not receive any other injuries which make them more susceptible to the radiation dose. Soldiers receiving less than a total of 150 cGy will remain combat effective. Between those two extremes, military personnel receiving doses greater than 150 cGy will become degraded; some will eventually die. A dose of 530-830 cGy is considered lethal but not immediately incapacitating. Personnel exposed to this amount of radiation will become performance degraded within 2-3 hours, depending on how physically demanding the tasks they must perform are, and will remain in this degraded state at least 2 days. However, at that point they will experience a recovery period and be effective at performing nondemanding tasks for about 6 days, after which they will relapse into a degraded state of performance and remain so for about 4 weeks. At this time they will begin exhibiting radiation symptoms of sufficient severity to render them totally ineffective. Death follows at approximately 6 weeks after exposure. Experiments conducted with animal models have shown that exposure to high doses of ionizing radiation (of the order of 25 Gy) results in an immediate precipitous decline in cerebral blood flow (CBF) which is followed by a partial recovery at 20-30 minutes, and subsequent slower secondary decrease in CBF thereafter accompanied by parallel changes in systemic blood pressure. These data indicate that radiation adversely affects the ability of the brain to regulate its blood supply. The implication of this indication extends into the realm of behavioral studies of early transient incapacitation and performance decrement (ETI-PD). The activity of certain brain enzymes involved in neurotransmitter metabolism is also considerably affected during ETI.
(3) Experimental results from animal studies indicate that, in general, partial body shielding reduces the behavioral effects of radiation. Head shielding is more effective in preserving the behavioral performance after exposure than is trunk shielding. Head shielding not only reduces the incidence of incapacitation but reduces the incidence of convulsions that normally accompanies early incapacitation. In all experimental causes studied to date, head shielding is most effective for doses in excess of 25 Gy.
a. Despite the high degree of radiosensitivity of some stages of germ cell development, the testes and ovaries are only transiently affected by single sublethal doses of whole-body irradiation and generally go on to recover normal function. In male test animals, low doses of whole-body irradiation cause abrupt decreases in sperm counts. The degree of decrease is dose dependent, but a transient azoospermia can appear at sublethal radiation doses. The resulting sterility may last several months to several years, but recovery of natural fertility does occur. The recovery depends upon the regeneration of those elements of the stem cell population which were in a relatively resistant part of the germ cell cycle. Other data suggest that under some conditions new spermatogonia may be formed by transformation from more radioresistant fixed stem cells.
b. When chromosome aberrations are produced in somatic cells, the injury is restricted to the specific tissue or cell system. However, when aberrations occur in germ cells, the effects may be reflected in subsequent generations. Most frequently, the stem cells of the germ cell line do not develop into mature sperm cells or ova, and no abnormalities are transmitted. If the abnormalities are not severe enough to prevent fertilization, the developing embryos will not be viable in most instances. Only when the chromosome damage is very slight and there is no actual loss of genetic material will the offspring be viable and abnormalities be transferable to succeeding generations. These point mutations become important at low radiation dose levels. In any population of cells, spontaneous point mutations occur naturally. Radiation increases the rate of these mutations and thus increases the abnormal genetic burden of future generations.
a. Recovery Processes.
(1) A variety of recovery processes may reduce radiation damage to a varying extent. For example, when a chromosome is broken, the broken ends tend to rejoin thus reconstituting the chromosome, but occasionally the broken ends seal over before rejoining thus leaving permanent chromosome damage. If two (or more) chromosomes are broken within the same cell, rejoining of inappropriate broken ends can occur and so may lead to permanent chromosomal change of a different kind. Repair of the broken ends of chromosomes, like all other repair processes following radiation damage, is not specific in respect of radiation damage. Repair is a biological process specific to a particular kind of damage which comes into play whatever the agent which causes that damage. These particular examples and others relating to DNA and its repair or to increased permeability of cell membranes, etc., are important in practice only for very large exposures.
(2) Three specific recovery processes are directly relevant to the medical aspects of defense operations. The first is an intracellular recovery within individual cells which have been sublethally irradiated. The second is a specific recovery of a specific tissue in which killed or damaged cells are replaced by division of surviving and minimally damaged or undamaged cells within that tissue, a process often called repopulation. Between them, these two processes may allow a complete return of function to normal. When the local dose is large enough, repair may be possible but incomplete. Repair of a specific tissue may be carried out without complete replacement of all the cells of the tissue. Healing may involve tissue atrophy and/or fibrosis and the irradiated tissue may be permanently scarred. The third, a combination of the first two types of recovery, can be very approximately quantified for lethality in humans by the use of the operational equivalent dose formula in cases where the irradiation period is protracted over several hours or longer as might happen in fallout conditions.
b. Intracellular Recovery. Individual irradiated cells have the ability to repair themselves as long as the amount of intracellular damage does not exceed a threshold value. The basic reason why sublethally irradiated cells survive and then recover is that a certain minimum amount of radiation energy must be deposited within a cell in order to kill it. Even when a mass of cells is uniformly exposed to low LET radiation, the amount of radiation energy deposited in individual cells is not the same for each cell but varies widely from cell to cell. As the dose increases, the proportion of cells increases in which a just lethal or more than lethal amount of energy is deposited. But all the other irradiated cells, those in which either no radiation energy or a sublethal amount is deposited, restore themselves to normal if given sufficient time to do so. Although controversial, it is generally believed that this mechanism for recovery is more effective in cells not undergoing active cell division, e.g., quiescent stem cells, than in cells undergoing active cell division, e.g., the basal cells of the intestinal crypts and the ordinary blast cells of the bone marrow. In quiescent cells, full recovery from sublethal radiation damage takes only a few hours. This can be demonstrated by dividing a dose into 2 fractions separated by a few hours when the damage observed will be less than when the whole dose is given all at once. This so-called Elkind repair continues during a protracted exposure to radiation, such as to fallout. It does not require a radiation-free interval.
c. Repopulation.
(1) Repopulation brought about by stem cell proliferation is a particularly important recovery mechanism in both the bone marrow and the gastrointestinal tract whenever the radiation exposure has been large enough to reduce cell numbers. Stem cells divide normally in both these tissues, because stem cell turnover is required to compensate for the normal continuously occurring removal of differentiated cells. Stem cell division can be accelerated by large doses of radiation. Large doses of radiation cause enough damage to stimulate this repopulation, just as any other severe insult would do. The effects of small doses are not recognized soon enough for accelerated proliferation to take place.
(2) In bone marrow, large microphage cells produce factors that either stimulate or shut down the stem cells that are the progenitors of the erythropoietic, granulopoietic, or thrombopoietic series of blood cells. The "factor producing" cells influence one another and depress the production of one factor while the opposite is being produced. Stem cell responses continue until the factor is changed. Some stem cells have the ability to cycle at faster rates than others but with lower efficiency, producing fewer mature cells eventually than the slower cycling cells. If the duration of exposure is sufficiently prolonged and the continued exposures are sufficiently large, then the repopulation process may become less efficient. However, it may take several months before the repopulation process becomes significantly impaired and so it is not likely to be relevant in a short duration nuclear warfare scenario.
d. Problems with Application of An Equivalent Dose Formula. It has long been realized that it is desirable to quantify recovery from ionizing radiation damage, especially when received more or less continuously over a period of time as would be expected when operating in fallout conditions. For operational reasons, the quantification needs to be relatively simple to use and should not require a computation with parameters that could not be established in a nuclear warfare scenario. Consequently, several equivalent dose formulas have been proposed which estimate the lethal dose from accumulated exposure. As such, these formulas can be used as guides to predict the levels of external exposure that could be tolerated from fallout fields. On the battlefield, however, they are of very limited use and could lead to serious overestimates of combat capability because they do not account for the effects of neutron exposures and predict only lethality, not radiation sickness, which could severely impair the effectiveness of combat personnel. Current equivalent dose formulas are applicable to a very small portion of a battlefield population, because they are valid only for external gamma doses received at low dose rates. Therefore, they cannot be used to predict the response of anyone exposed to neutrons. This limitation renders the formulas unusable for any military personnel irradiated at the time of a nuclear detonation since neutron dose is known to be more lethal than a comparable dose of gamma radiation alone. Present formulas potentially would be applicable only to forces being introduced into a fallout field after the cessation of nuclear detonations. Their practical use on the battlefield is further reduced by NATO and enemy nuclear targeting doctrine which call for detonations at altitudes that preclude the generation of fallout and by the difficulty in predicting arrival of fallout fields. Given the small range of application to the nuclear battlefield and the possible errors they might cause, current equivalent dose formulas are inappropriate for operational decision making on the nuclear battlefield.
Late or delayed effects of radiation occur following a wide range of doses and dose rates. Delayed effects may appear months to years after irradiation and include a wide variety of effects involving almost all tissues or organs. Some of the possible delayed consequences of radiation injury are life shortening, carcinogenesis, cataract formation, chronic radiodermatitis, decreased fertility, and genetic mutations.
a. Irradiation of almost any part of the body increases the probability of cancer. The type formed depends on such factors as area irradiated, radiation dose, age, and species. Irradiation may either increase the absolute incidence of cancer or accelerate the time or onset of cancer appearance, or both. There is a latent period between the exposure and the clinical appearance of the cancer. In the case of the various radiation-induced cancers seen in mankind, the latency period may be several years. Latency as well as the dose required to induce cancers varies with the cancer site and with the species studied. Latent periods for induction of skin cancers in people have ranged from 12 to 56 years after x irradiation therapeutic exposures with estimated doses of several thousand roentgens. Fifteen years is reported as a latent period for bone tumors from radium. This latency related to bone tumors is very dependent upon the dose and type of radiation emitted by the radionuclide.
b. A leukemogenic effect was expected and found among Hiroshima and Nagasaki survivors. Peak incidence occurred 6 years after exposure and was less marked for chronic granulocytic leukemia than acute leukemia. The incidence was inversely related to distance from the hypocenter. British persons receiving radiotherapy for spondylitis showed a dose response relationship for leukemia, with peak incidence occurring 5 years after the first exposure. Studies have demonstrated that ionizing radiation can induce more than one kind of leukemia in people, but not chronic lymphocytic leukemia.
c. Predisposing factors for tumor development include heredity, age, hormones, and prior exposure to physical trauma, chemical agents and ionizing radiation. The actual processes by which cancer is induced are not known. Somatic mutations, virus infections, and precancerous abnormalities in tissue organization and vascular supply have all been postulated.
A late effect of eye irradiation is cataract formation. It may begin anywhere from 6 months to several years after exposure. While all types of ionizing radiation may induce cataract formation, neutron irradiation is especially effective in its formation, even at relatively low doses. Cataract formation begins at the posterior pole of the lens and continues until the entire lens has been affected. Growth of the opacity may stop at any point. The rate of growth and the degree of opacity are dependent upon the dose as well as the type of radiation. The threshold for detectable cataract formation in 2 Sv (sievert) (200 REM (roentgen equivalent, man)) for acute radiation doses and 15 Sv (1500 REM) for protracted doses.
Delayed, irreversible changes of the skin usually do not develop as a result of sublethal whole-body irradiation, but instead follow higher doses limited to the skin. These changes are a common complication in radiation therapy but they should be rare in nuclear combat unless there is heavy contamination of bare skin with beta emitter material from fallout, in which case beta-induced skin ulceration could be seen. The condition should be easily prevented with reasonable hygiene and would be particularly rare in climates where the soldiers were fully clothed (arms, legs, and neck covered). Table 5-V lists the degrees of radiation dermatitis for various radiation doses.
a. When radioactive materials are incorporated into the body and retained, significant radiation injury can be sustained by specific tissues in which the materials are concentrated or in some instances by the whole body. The primary factors which determine the type and degree of injury are the types and amounts of the isotopes deposited and the nature and energies of the radiation emitted.
b. Each isotope follows a fairly specific biological pathway in the body. This pathway may be quite complex with several compartments and is determined by the chemical nature of the isotope. A given isotope may be concentrated or retained in a specific organ or tissue during the time it is in the body. It may be eliminated from the body, and the rates of elimination of different isotopes vary considerably. More than one isotope may be incorporated in the body at the same time, and the effects of a mixture of isotopes found in fallout would be additive.
c. In this section, certain basic principles and factors governing isotopes in the body are discussed; these include their distribution, action, and elimination. The associated clinical problems are discussed in Chapter 6.
The basic routes of entry for isotopes are: inhalation, ingestion, and absorption through the skin. Following ingestion or inhalation, a given material may be absorbed into the blood stream, depending upon its volubility. Insoluble materials are not absorbed, except in extremely small amounts, and may be eliminated fairly rapidly directly from the respiratory and gastrointestinal tracts. However, under certain circumstances, insoluble materials can be retained at or near the original site of deposition, e.g., in the lungs or in wounds, or may be translocated to regional lymph nodes, where again they will constitute an internal radiation hazard. Only the very small particles of radioactive materials, 10 microns in diameter or smaller, are deposited in the alveolar airsacs.
a. Inhalation.
(1) An insoluble material which is inhaled in the form of an aerosol will be deposited along the tracheobronchial tree. Much of it will be removed by the ciliary action of the mucosa lining most of the respiratory system, but a certain fraction, depending on the size, shape, and density of the particles, will penetrate down to the alveolar airsacs and remain. Only the very smallest particles penetrate that far; and so, the percentage of inhaled insoluble particles which are retained in the lungs is small, generally less than 25%. However, material so retained can be a considerable hazard to the lung, since it may remain for a long time. A portion of this material will be picked up by the lymphatic system draining the various pulmonary regions. It will then be collected by and remain in the lymph nodes of the lungs and still be a longterm hazard to lung tissue. A small fraction of the material may reach the blood stream and end up trapped in the reticuloendothelial system in various regions of the body and for certain isotopes, such as plutonium and strontium, also in bone.
(2) If a soluble material is inhaled, it is absorbed very rapidly and completely, and often will not remain in the lungs long enough to cause significant damage. Once in the circulation, it will be distributed in the body in the same way as it would following any other mode of entry.
b. Ingestion.
(1) An insoluble material which is ingested will remain in the gastrointestinal tract and become mixed in and part of the fecal material in the large bowel, with which it will then be eliminated. This includes swallowed material cleared from the upper respiratory tract and the tracheobronchial system by ciliary action. Insoluble material is not retained in the gut as it is in the lungs or in soft tissues, and the radiation hazard is limited in time to that required for transit and elimination, generally a matter of hours. As a result, the radiation hazard is negligible, unless the material includes a highly active gamma emitter. Normally, beta and alpha radiation from insoluble radioactive material in the gut lumen will not cause significant damage. The few cells of the mucosa which are damaged slough off and are replaced rapidly. A gamma emitter on the other hand would be a whole-body hazard as long as it was in the gut. Highly radioactive fallout containing fission products emitting beta and gamma radiations could cause some gastrointestinal tract damage if accidentally ingested with contaminated foodstuffs or water. However, in most such instances, the whole-body exposure received from external gamma radiation in the area would be the controlling hazard.
(2) When a soluble material is ingested, absorption is quite efficient. This is the most significant route of entry for the soluble isotopes in fallout, particularly when fallout-contaminated water or food is consumed. A number of fission products can become incorporated into vegetation and enter into complex food chains. In some instances, certain radioactive materials can be concentrated in these chains increasing the eventual hazard to humans.
c. Transcutaneous Absorption.
(1) An insoluble material contaminating the intact skin can be an external hazard only if it is a gamma or beta emitter. It will not be absorbed into the blood stream and thus will not become an internal hazard. Conceivably, contamination of the skin with large quantities of gamma emitting materials could result in significant whole-body irradiation. This could occur when personnel have been subjected to heavy fallout contamination. However, this can be easily prevented by prompt removal of contaminated clothing and washing exposed areas of skin. If a wound is contaminated, insoluble material will tend to remain localized in the tissue at the wound site, unless removed by debridement. Some would be present within the eschar. This type of contamination should not cause a serious problem, unless it is particularly high in radioactivity. A small but measurable fraction of the material will be cleared from the wound site by lymphatic drainage. Most of this material will be trapped in the regional lymph nodes which drain the area of the wound, similar to that process described for the lungs.
(2) Soluble material will be absorbed readily through wound sites and distributed within the body organs and tissues according to the usual metabolism of the stable isotope of the element in question. Some soluble materials, particularly tritium, will be absorbed rapidly and totally across the intact skin.
a. A radioactive material must be eliminated from the body to remove its hazard. Detoxification, which is effective against materials which are chemical hazards, will not be effective since radioactivity is not modified by chemical changes. The methods of elimination include renal excretion for most soluble materials, elimination in the feces for materials which are retained in the gut or which can be secreted in the bile, and exhalation for volatile materials and gases. Chelating agents, e.g., calcium or zinc DTPA (diethylenetriamine pentaacetic acid), if administered soon after exposure, are effective in enhancing the elimination of certain radioisotopes. These materials are not very effective for radioisotopes which have been incorporated and fixed in organs and tissues, e.g., bone. Under conditions of nuclear war, chelation therapy is very unlikely to be used. (See 717e.)
b. The rate at which a material is eliminated is usually expressed as the biological half-life. This is the time it takes for one-half of a given amount of material to be excreted or eliminated. During each successive half-life, an additional one-half is removed from the body. It is analogous, therefore, to the physical half-life. Not all materials follow a simple exponential elimination process, but this method of expression is sufficiently accurate to be applicable to most soluble isotopes. An exception which must be noted is the retention of insoluble heavy metals such as plutonium in the lungs and in bone. The rates of loss under these circumstances are not exponential and are very slow.
c. The biological half-time may be variable. A prime example of this is body water, the turnover of which can be as short as 4 days to as long as 18 days depending upon the state of hydration, volume of intake, and renal function. If tritiated water is incorporated into the body, the biological half-life is the factor determining the hazard since it is so much shorter than the physical half-life of about 12 years. Reduction of the biological half-life to a minimum by overhydration and the administration of diuretics has obvious value and is the recommended therapy in cases of exposures to tritium. Other isotopes cannot be cleared from the body as rapidly, and there is no adequate treatment available at present for increasing the rate of removal of a mixture of isotopes which would be incorporated into the body as a result of ingesting fallout contaminated food and water.
d. The overall hazard of materials which are eliminated exponentially will be a function of their physical and biological half-lives considered together. Whichever is shorter will become the primary factor. The effective half-life is usually determined and expressed by the following formula:
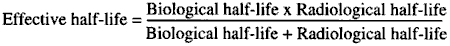
e. The uptake by the body of radioisotopes can be blocked in some cases. For example, potassium iodide or iodate if given prior to or soon after an intake of radioiodine, will reduce the uptake of radioiodine by the thyroid gland. Similarly, orally administered Prussian Blue will reduce the absorption of cesium from the gut and Alginate will reduce strontium absorption. No policy exists which would allow for NATO forces to stock and issue chelators.